Molecular Geometry of Thionyl Chloride Socl2 Is Best Described as
The lewis structure of a molecule is also known as its electron dot structure. Note that rounding errors may occur so always check the results.

Geometry Of Molecules Socl2 Lewis Structure Thionyl Chloride Facebook
So perhaps due to the high degree of ionic character in the ceS-Cl and ceS-O bonds the missing two electrons are dispersed among these ligands.
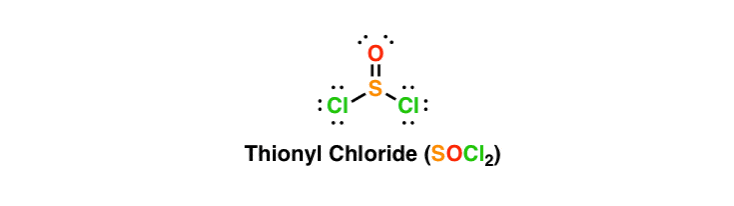
. The Molecular geometry of thionyl chloride SOCI2 is best described as 1 See answer Advertisement Advertisement asikagi asikagi According to Lewis Structure there are three 3 atoms attached to the central sulfur and a lone pair of electrons on central sulfur. Here we will provide a irà explicaÃà the molecular geometry. Connect the atoms of SOCl2 with single bonds.
And that equals 26 valence electrons. 7 rows The molecular geometry of SOCl2 is a trigonal pyramid because the central atom of it has one. Then because there are four areas of electron density with one lone pair the shape is trigonal pyramidal.
Oxalyl chloride tends to be bit more expensive but often. Sulfur has 6 valence electrons. Aug 17 2021 The molecular geometry of SOCl2 is trigonal pyramidal and its electron geometry is tetrahedral.
Postby Clara Hu 1G Thu Nov 30 2017 642 pm. SOCl2 H2O SO2 2HCl. Lets do the SOCl2 Lewis structure.
SOCl2 is pyramidal in shape this is because sulphur being the central atom has 6 electrons in its valence orbit out of that six electrons 2 are is S orbital and the rest are in P orbital. Charge is conserved and thionyl chloride should always have 26 electrons no matter how they are distributed. Use this page to learn how to convert between moles Thionyl Chloride and gram.
Click to see full answer Consequently what is the point group of h2o2. Upon further thought this still makes no sense. Copy Sheet of paper on top of another sheet.
1 mole is equal to 1 moles Thionyl Chloride or 1189704 grams. We have 26 valence electrons. Thionyl chloride SOCl2 hypochlorous acid HClO are examples of Cs molecular symmetry point groups which can undergo an E σh symmetry operation have no mirror planes or other symmetries.
Convert grams SOCl2 to moles or moles SOCl2 to grams Molecular weight calculation. SOCl 2 adopts a trigonal pyramidal molecular geometry with C s molecular symmetry. 1 Answer to The molecular geometry of thionyl chloride SOCl2 is best described as A trigonal planar B T-shaped C tetrahedral Dtrigonal pyramidal E linear Home Questions.
The molecular formula for Thionyl Chloride is SOCl2. Choose the best Lewis structure for SOCl2. The central atom Sulfur will have 3 bonding pairs and 1 lone pair.
This geometry is attributed to the effects of the lone pair on the central sulfur IV center. Lets put a Chlorine on either side and then the Oxygen right there. The molecular geometry of thionyl chloride SOCl2 S O C l 2 is best described as.
PCl5 SO2 POCl3 SOCl2. The molecular geometry of thionyl chloride SOCl2 is best described as A trigonal planar B T-shaped C tetrahedral Dtrigonal pyramidal E linear. You can use oxalyl chloride instead of thionyl chloride.
Structure of SOCl2 SOCl2 adopts a trigonal pyramidal molecular geometry molecular symmetry C. How to Draw the Lewis Structure for. Thionyl chloride is used as an oxidizing and chlorinating agent in organic chemistry.
Thionyl chloride SOCl2 or Cl2OS CID 24386 - structure chemical names physical and chemical properties classification patents literature biological. Enter the email address you signed up with and well email you a reset link. The SI base unit for amount of substance is the mole.
SO2 2Cl2 SOCl2 Cl2O. Molecular weight of SOCl2 SOCl2 molecular weight Molar mass of SOCl2 1189704 gmol This compound is also known as Thionyl Chloride. Hydrogen Peroxide C Hydrogen Peroxide only contains a C2 axis.
Chemistry questions and answers. With two pairs of ligaÃÃ o two pairs solitÃ. In the solid state SOCl 2 forms monoclinic crystals with the space group P2 1 c.
For SOCl2 the oxygen atom and two chlorine atoms are each bonded to sulfur by a single bond and sulfur also has a lone pair to complete the octet. Chlorine has 7 we have two Chlorines. 32065 159994 354532 Percent composition by element Calculate the molecular weight of a chemical compound.
Both TA sà the right as wrong or as Lewis structure of thionyl chloride. 51 The Covalent Bond Model 52 Lewis Structures For Molecular Compounds 53 Singel Double And Triple Covalent Compounds 54 Valence Electrons And Number Of Covalent Bonds Formed 55 Coordinate Covalent Bonds 56 Systematic Prodedures For Drawing Lewis Structures 57 Bonding In Compounds With Polyatomic Ions Present 58 Molecular Geometry 59. Select the best Lewis structure for SOCl2.
Sulfurs the least electronegative it can go at the center.

Reagent Friday Thionyl Chloride Socl2 Master Organic Chemistry
What Is The Shape Of Socl2 Quora

Socl2 Lewis Structure How To Draw The Lewis Structure For Socl2 Youtube
No comments for "Molecular Geometry of Thionyl Chloride Socl2 Is Best Described as"
Post a Comment